Components and Physical Layout

The gel electrophoresis and transilluminator module is on the left side of the Bento Lab system.
The gel box is made up of two parts. The base (2) is made of clear acrylic. It is used to cast gels and as a gel tank during electrophoresis. It also stores casting shutters and combs. The lid (1) is made of orange acrylic that functions as a filter for the blue light transilluminator. On the left side of the Bento Lab system (3), a red and black socket provide current for gel electrophoresis. The blue LED transilluminator (4) is located on the left side of the device.
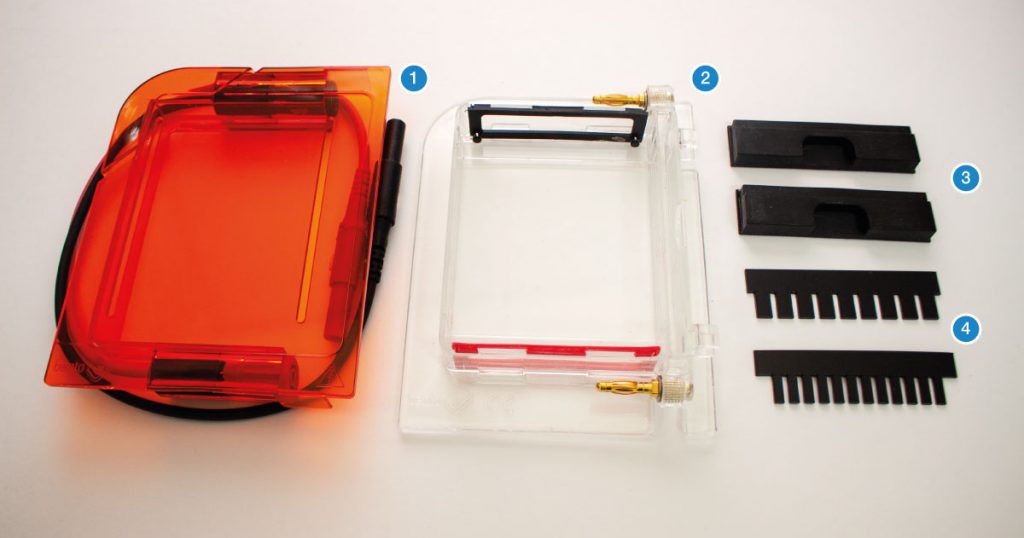
The gel box contains several parts, including the orange lid (1) and the gel tank (2), two dams used to create buffer zones during gel casting (3), and combs for 9 and 12 well gels (4).
Opening the gel box
Compared to other gel boxes, the gel box has a novel opening mechanism: the lid slide opens.
To open the gel box, slide the orange lid away from the gel tank.

Specifications
| Voltage | 50V – 120V |
| Box Dimensions | 91mm x 78mm |
| Visualisation | 470nm blue light and diffuser |
| Viewing Window | As box dimensions |
| Filter | Amber acrylic |
User Interface
To use the Gel Electrophoresis and Transilluminator module, select it from the home screen.
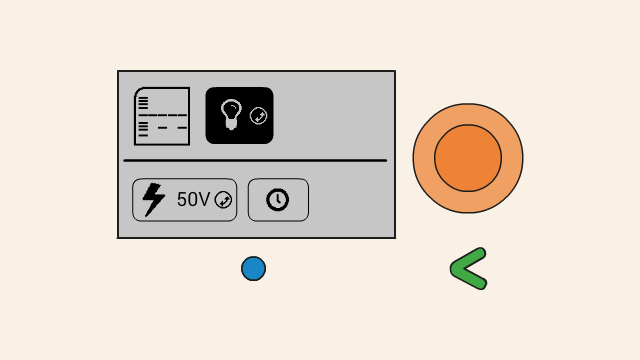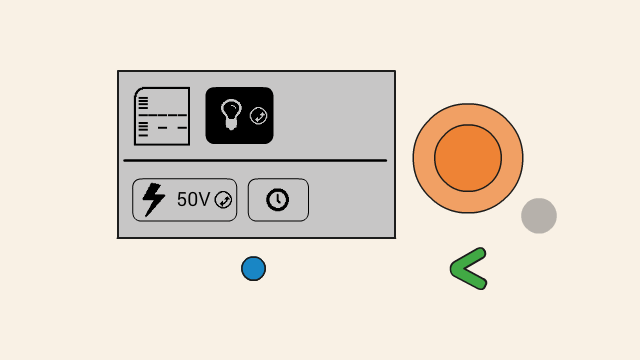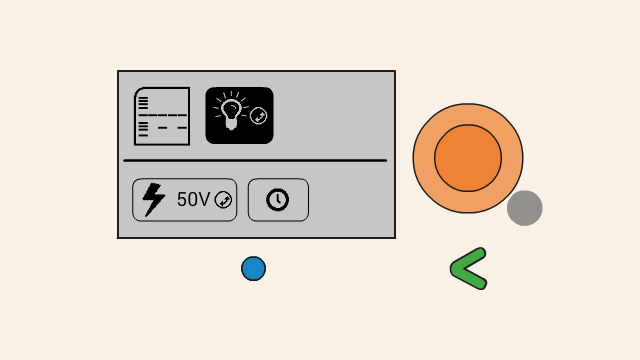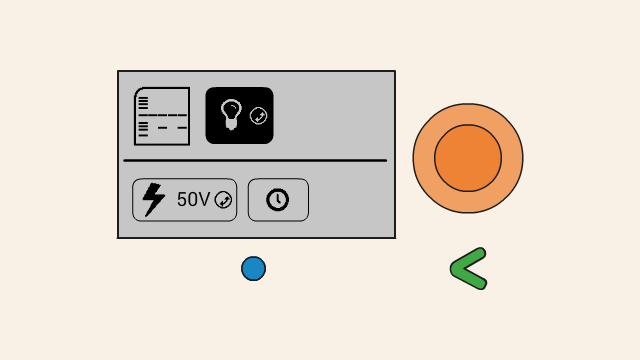
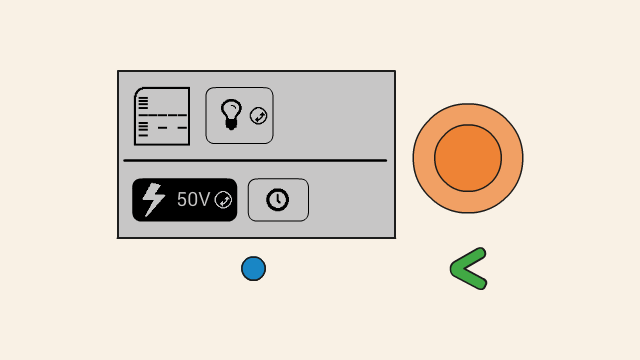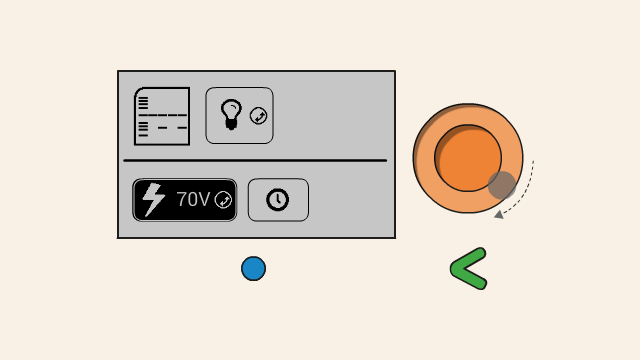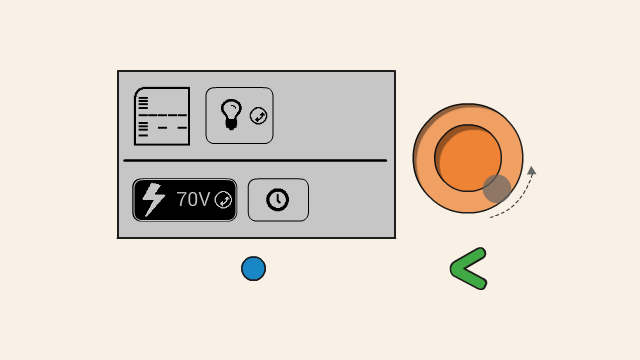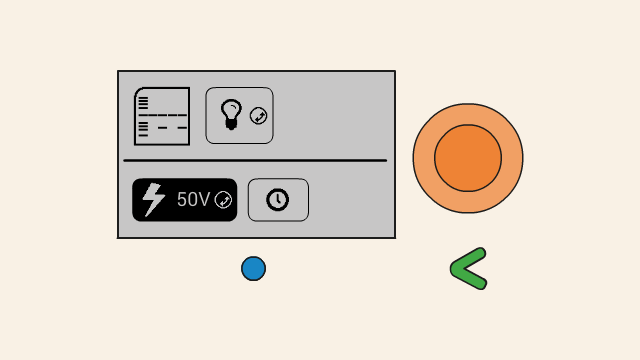
To set the voltage of a gel, first select the voltage button. Push down the orange click-wheel and keep holding it down while rotating it to change the value. Dial clockwise to increase the voltage, and counter-clockwise to decrease it.
To start running the gel at the selected voltage, press the timer button. On the next screen, set the timer. To change the duration, push down the orange click-wheel and keep holding it down while rotating it to change the value. Dial clockwise to increase the duration, and counter-clockwise to decrease it.
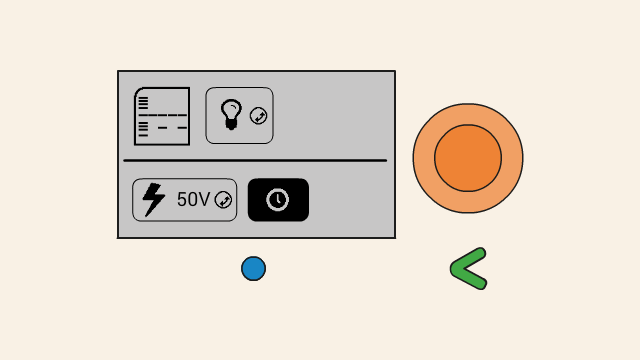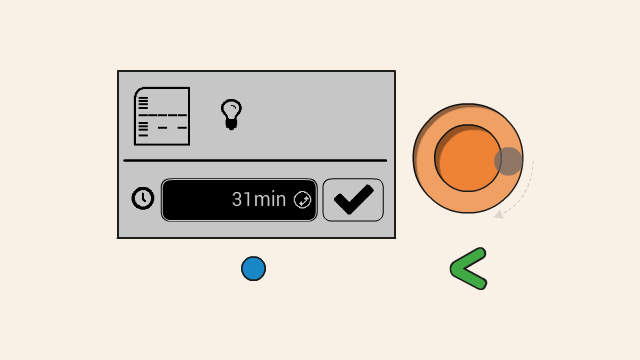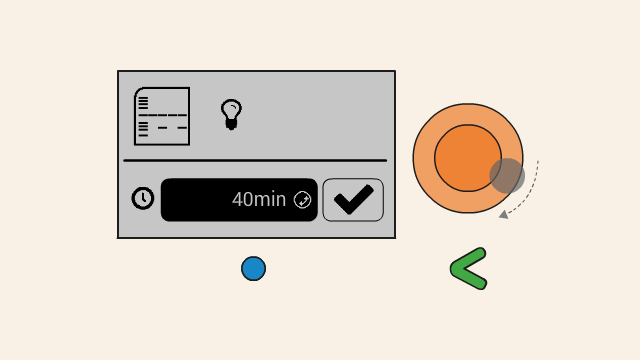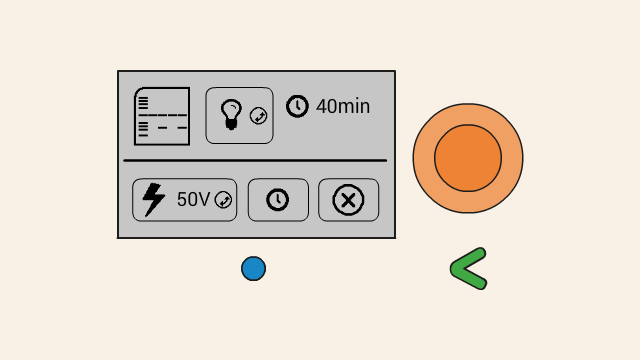
Make sure your gel is ready and connected to Bento Lab before hitting the confirm button.
If Bento Lab does not detect a current draw, or if the current draw is too high, an error message will be displayed. If no current is detected, despite the gel box being connected, this might indicate a broken platinum wire.

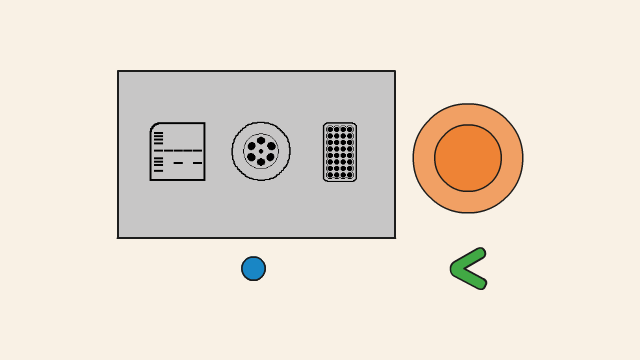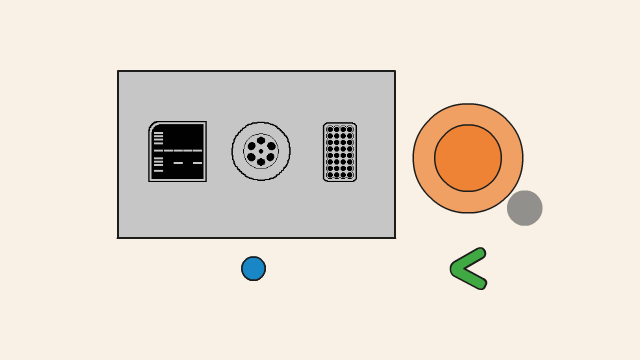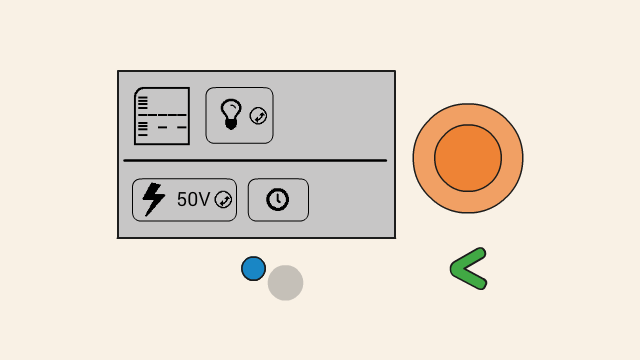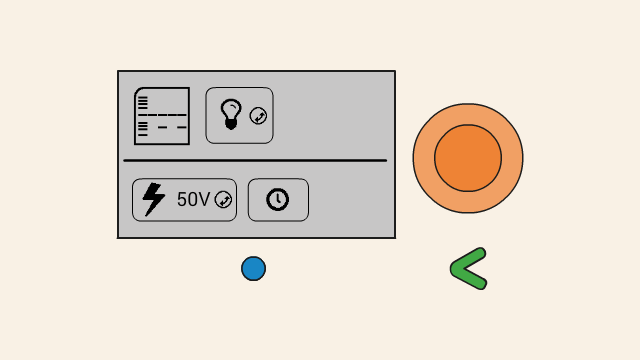
When the gel is running, the remaining time is displayed on the top right position (1), and a flashing lightning bolt indicates that current is flowing (2). The clock button (3) allows for the timer to be adjusted, and the cancel button (4) can be used to turn off the power to the gel.
When the gel has finishing running, either:
- (without dark box) unplug the gel tank and place it carefully on top of transilluminator, or
- (with dark box) unplug the gel tank, carefully remove the orange lid and place the gel tank (containing the gel and TBE buffer) on top of the transilluminator and place the dark box over it.
Select the light bulb button and press the orange click-dial to toggle the transilluminator on and off. The gel can be viewed safely through the orange lid or orange eye hole on top of the dark box.
Warning: When the transilluminator is on, do not stare directly into the blue light for prolonged periods of time.
The light intensity can also be modulated by holding the click-dial down and rotating it.
Casting a gel
All Bento Lab protocols use 0.5X TBE buffer in the gel electrophoresis step. We recommend preparing a stock solution of 0.5X TBE buffer by adding 50 mL of 10X TBE buffer to 950 mL of deionised water in a glass or plastic bottle.
- Pour out required amount of 0.5X TBE buffer into a microwaveable container and add agarose tablet(s) according to the protocol you are following. For a 1% gel add 1 tablet to 50 mL of buffer (1.5% gel is 1 tablet in 33ml buffer; 2% gel is 2 tablets in 50ml buffer; 3% gel is 2 tablets in 33ml buffer.)*
- Wait for ~2 mins until the tablet has disintegrated, then heat the solution in a microwave at full power for short bursts of 20-30 seconds. Swirl the solution in between bursts to mix, until the agarose is completely dissolved. It should be completely clear with no traces of any particles (incompletely melted agarose will result in poor amplicon visualisation). If particles are present then microwave briefly again until the agarose is clear. Avoid overboiling the mixture, as the buffer will evaporate and your gel will have a higher concentration of salts than the running buffer (which may cause poor migration and overheating) and you will end up with a higher concentration of agarose than desired.
- Check you are working on a level surface to cast a gel with uniform thickness.
- Gently slide the orange lid off the gel tank.
- Place the dams in the tank to cover the buffer zones around the electrodes. Wetting the dams with tap water before inserting them can help provide a better seal.
- Select a comb and insert the comb into the holder.
- Once the agarose has cooled to about 55°C, it should feel hot, but not too hot to touch. At this point add the recommended volume of DNA stain (this will depend on the type of DNA stain you use) and swirl gently to mix. For GelGreen®, 1 μL per 10 mL of TBE buffer is recommended, but as little as 0.5 μL per 10 mL can also produce good results and save on costs. Then carefully pour the melted gel into the gel tank.
- Check the agarose gel for bubbles. If any bubbles are present, these can be popped or drawn to the bottom of the gel with a pipette tip.
- Allow the gel to cool down and solidify. This takes about 30 min at room temperature, or 15 min in a cool room or a fridge.
- Gently remove the bottom dam first and make sure that the gel is completely set and has left a sharp edge. If it hasn’t fully set then wait another 5-10 minutes. Then remove the top dam and comb.
- Inspect the gel for integrity. The gel should have sharp edges at the top and bottom, and the wells should be well-formed. In the rare occurrence that there has been a leak due to a dam not being securely fitted, a thin film of agarose may have set between the gel and the electrode. This can be carefully cut and removed with a pipette tip, taking care not to damage the electrode.

What percentage of agarose should I use?
All Bento Lab protocols recommend which percentage agarose gel to use to give the best visualisation of the PCR amplicons expected from the experiment.
The greater the percentage of agarose, the smaller the fragment of DNA that can be resolved. The appropriate percent agarose gel depends on the size of DNA fragment that you will be running on the gel. You will obtain good resolution of larger DNA fragments (5-7kb) with a 1% agarose gel, whereas for good resolution of small PCR products, you will need a higher percent gel.
| DNA size resolution | Percent agarose gel | Agarose tablet(s) in 0.5X TBE buffer |
| 1-30 kb | 0.5% | 1 tablet per 100 mL |
| 800 bp – 12 kb | 0.7% | 1 tablet per 71 mL |
| 500 bp – 10 kb | 1.0% | 1 tablet per 50 mL |
| 400 bp – 7 kb | 1.2% | 1 tablet per 42 mL |
| 200 bp – 3 kb | 1.5% | 1 tablet per 33 mL |
| 50 bp – 2 kb | 2.0% | 2 tablets per 50 mL |
Running a gel
Bento Lab contains a mini gel electrophoresis system, therefore a higher voltage gradient than larger gel systems, as the distance used to determine voltage gradients is the distance between the electrodes, not the gel length. If the voltage is too high, you may observe band streaking. For best results, we recommend running gels at 50V with 0.5X TBE.
- Pour the 0.5X TBE buffer solution into the gel tank over your prepared gel until the liquid covers your gel, and the level is about 2-3 mm above your gel.
- Carefully load a molecular weight ladder into the first lane of the gel.
- Load your samples into the remaining wells of the gel.
- Gently slid the orange lid onto the gel tank until it clicks shut.
- Set the voltage to the desired electrophoresis voltage for your protocol. To make changes to the voltage, select the voltage value button. Push down the orange click-wheel and hold down whilst rotating to change the value. Dial clockwise to increase the voltage, and counter-clockwise to decrease. Release the orange click-wheel once you have reached the desired voltage.
- Set the desired time by selecting the time value, pushing down and rotating the orange click-wheel.
- Confirm your settings by clicking on the tick on the right, and set the gel to run.
- Run the gel until the dye line has travelled down about 75% the length of the gel. A typical run time with Bento Lab is about 30-45 minutes, depending on the gel concentration and voltage.
- Wait until the run has finished, or cancel the run. Disconnect the electrodes from the power source, and then carefully remove the gel from the gel box.
- Place your gel on the transilluminator, and select the transilluminator button to turn the blue light on to visualise your bands.